Kangaroo Mother Care: A Case Study on KMC’s Beginnings and its Worldwide Implementation (Part II)
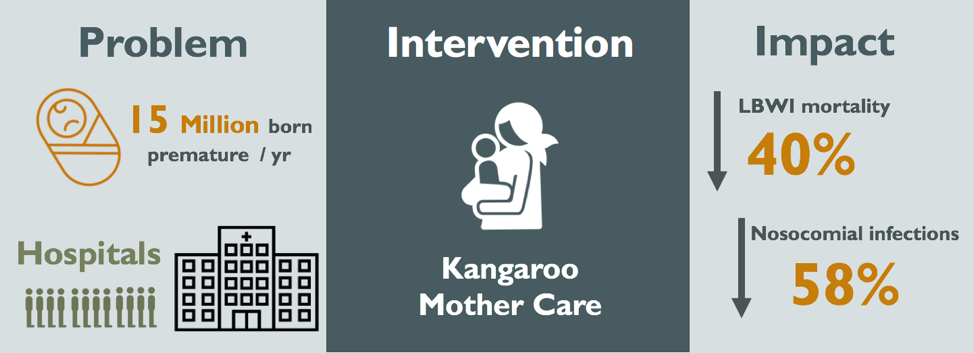 Figure 1: Visual Abstract of the Kangaroo Mother Care Method
Figure 1: Visual Abstract of the Kangaroo Mother Care Method
Editor’s Note: This article was written by Courtney Fang, Zoë Goldstein, Morgan Kennedy, Carla Achcar, Bidjinie Coriolan and Mackenzie Hamilton, for a case study in PPHS 511: Fundamentals of Global Health. Read Part I of this piece here.
Successes and Barriers Experienced in the Implementation of KMC
KMC is an important “example of both South-South and South-North knowledge transfer” in evidence-based medical care, and its benefits have been observed across settings (Bergh, 2007). Since its inception in 1978, extensive evaluative research was conducted by the Fundación Canguro, and a formal training program for other large public hospitals in Colombia and developed countries was initiated in 1994 (Rosero, n.d.). The model of KMC dissemination is “see one, do one, teach one” (the SODOTO model): health care leaders are invited to the Fundación Canguro center for training (“see one”), to run a KMC program in their home institution (“do one”) and then train others in their region for further KMC implementation (“teach one”) (N. Charpak, personal communication, 7 December 2018). Knowledge transfer has been slow but successful in Colombia, as practices are culturally acceptable and effective. Nearly all the teams trained in Bogotá successfully set up programs, despite limited equipment. At present there are about 35 regional KMC programs in public and private institutions offering the same “KMC package” that is compatible with health ministry rules (N. Charpak, personal communication, 7 December 2018).
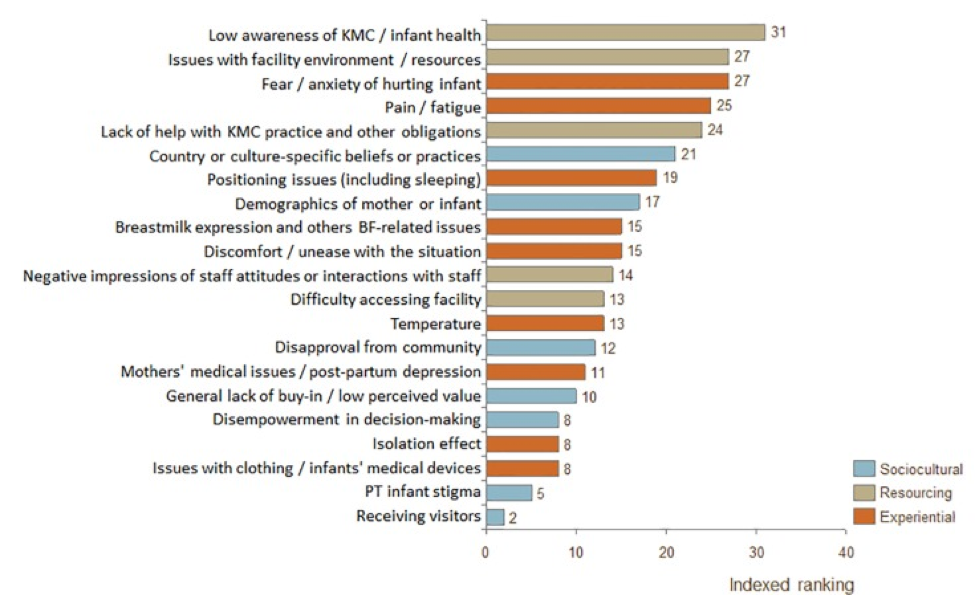
Characteristic of any new programming or change to standard practice, challenges were faced in KMC’s introduction and implementation. As reported by Fundación Canguro, KMC had to overcome initial resistance from healthcare workers and difficulties regarding appropriate discharge and follow-up procedures (Rosero, n.d.). Specifically, nurses had initial distrust regarding mother’s competence to care for the child at home and neonatologists resisted changes to routine medical practices. A summary of barriers faced during adoption of KMC in low and middle income countries can be seen in Figure 1 of the Appendix. To address concerns and challenge perceptions that KMC may increase hospital workload, staff were appropriately trained and educated, and an intrahospital kangaroo adaptation unit was created (Rosero, n.d.). In the adaptation unit, staff trained mothers on KMC and evaluated discharge eligibility. Discharge criteria were essential for combatting administrative pressures to release infants early from overcrowded facilities.
Additionally, advocacy was needed at the policy-level for Social Security coverage. Several studies determined that early detection of visual, auditory, or motor development delays would prevent higher cost interventions and burdens in the long-run, thus justifying minimal year follow-up for high risk cases (Rosero, n.d.). By properly educating and training stakeholders with available evidence, implementers and advocates addressed misinformation and negative perceptions regarding KMC. Importantly, the core elements of KMC delivery—position, feeding, and early discharge—were culturally appropriate per local customs and religion for initial roll-out (Rosero, n.d.).
Scholars and practitioners have further investigated barriers and enablers to KMC implementation across contexts to develop appropriate recommendations (Cattaneo, 1998; Charpak & Ruiz-Peláez, 2007). It is seen that in addition to healthcare barriers faced, sociocultural factors exist and may impede successful take-up of KMC in other settings at varying degrees. The requirement of diapers in settings where it is not traditional practice, mothers’ limited privacy and resulting exposure, perceptions of extra work for staff, restricted participation of father, discomfort of the mother, lack of using caps and socks in warm climates and the effect of continuous skin-to-skin contact on mother’s other duties or daily routine, have all been identified as practical barriers (Charpak & Ruiz-Peláez, 2007; Chan, 2015; Chan, 2017). For example, KMC’s interference with “obligations related to mother’s daily routine” was reported from Zimbabwe, Uganda, and Guinea (Chan, 2015). Fundación Cangaru has received more than 35 developing countries for KMC training; however, there has been special difficulty with northern Africa. One interpretation is that the promotion of the active role of the father may be unattractive or difficult to put into practice in settings where pregnancy, newborn care, and the first years of life are private property of the women (N. Charpak, personal communication, 7 December 2018).
Additional factors identified in the literature that influence KMC implementation include buy-in, support and empowerment, time, medical concerns, access, and cultural norms (Chan, 2017). Therefore, coordinated actions and consistent communication among national and local leadership is needed to support essential health systems and financing requirements for KMC (Chan, 2017).
Is KMC Cost-Effective?
Given the simplicity of KMC, a common misconception is that little funds are needed to effectively and appropriately administer the treatment. Parents become major caregivers of the infant, leading to decreased economic productivity. Furthermore, implementation requires parent coaching and frequent follow-up after discharge in specialized clinics. Still, the analyses performed on implementation all over the world have been promising.
Observational studies of implementation in 12 Nicaraguan facilities estimate that US$230 000 would be saved annually (Broughton, 2013) by formally instituting KMC. Further studies on implementation in India estimated that hospitals saved US$570 per LBW infant treated with KMC compared to traditional intermediate intensive care (Sharma, 2017). Most importantly, an incremental cost utility analysis on a randomized controlled trial in Bogotá, Columbia proved that KMC is significantly more effective and less costly than traditional care (Ruiz, 2017). While these results are specific to regional hospitals and their systems in place, the trend remains the same all around the world. Moreover, these results likely underestimate the cost-effectiveness of KMC. 20 years after its first implementation, we are seeing KMC significantly increase brain development and social skills – both of which have potential impact to improve economic productivity (Charpak, 2017).
Where is the money coming from?
Two of the United Nations’ eight Millennium Development Goals (MDGs) focus on improving maternal health and reducing child mortality. This era of MDGs has helped mobilize funding in areas to aid with the implementation of KMC. For example, USAID and their Healthcare Improvement Project (HCI), in coordination with the Ministry of Health in Nicaragua, have been heavily involved in the implementation of KMC in rural hospitals of Nicaragua (Broughton, 2013). Beyond 2015, there has been continued support in the form of programs such as the “Every Newborn Action Plan,” which aims to treat 50% of low birth weight infants with KMC by 2020 (Every Newborn: an action plan to end preventable deaths, 2014). This plan has been largely funded by the Global Financing Facility (GFF) trust fund, which was initiated with a pledge of $875 million from the governments of Canada and Norway, as well as the Bill and Melinda Gates Foundation. Since its initiation, many other foundations have supported this trust fund, such as GAVI, The Vaccine Alliance, The World Bank Group, and other bilateral agencies (Reaching the Every Newborn National 2020 Milestones, 2017). Overall, their goal is to mobilize over $18 billion in funding between now and 2030 (The Global Financing Facility 2016-2017 Annual Report, 2017).
While these external agencies have limited funding, the simplicity of KMC offers unique sustainability if implemented correctly. As seen in Nicaragua, involving national health agencies in the implementation allows for the development of sustainable, ‘horizontal’ plans to maintain proper care.
Future Implications
Scale-up from Colombia to Asia and Africa – the difficulties experienced with KMC implementation:
In Latin America, since its inception at the Maternal Infant Institute (IMI) of Bogotá, Colombia in 1978, scale-up has been achieved through the SODOTO model of dissemination. As discussed above, teaching has been utilized to disperse the knowledge on KMC in most Latin American countries and certain European hospitals (Bergh, 2012). This model of dissemination is also being used to implement KMC in sub-Saharan Africa and South Asia (Bergh, 2012; Bergh, 2016), where 60% of all preterm births occur (Blencowe, 2013). These regions would benefit the most from implementation of the KMC method; however, uptake has been less than optimal.
Several aspects of implementation strategies explain this lack of progress. The model of dissemination engenders intermittent funding, usually from non-governmental organizations (Bergh, 2016). Dependence on these donors can seriously halt the growth in implementation of KMC. Furthermore, a shortage of advocacy leaders in these countries hinders the spread of the intervention from lone facilities to a more organized scale-up at a national level.
The majority of African healthcare systems are overwhelmed with a high burden of infectious diseases combined with the growing reality of non-communicable diseases. Moreover, financial decision-makers are not involved enough in discussions about mother and child health and are therefore unable to request more resources for KMC for its scale up in the health-care systems (Bergh, 2016). The SODOTO model of dissemination comes with good intentions, but generates vertical KMC programs that are too expensive to sustain. Stakeholders need to propose ways in which KMC can be implemented in the existing systems of care for newborns (Bergman, 2015).
Is facility-based KMC sufficient for impact?
Although there is a consensus that hospital-based KMC is effective and sustainable, there are still questions surrounding the true impact of this approach. Most births in South Asia, Southeast Asia, and sub-Saharan Africa take place at home. In the two lowest wealth quintiles, more than 70% of all births are home deliveries, and at least half of them are unattended (Montagu, 2011). The potential impact of facility-based KMC fades when keeping these numbers in mind. Facility-based care is important, but to tackle the bulk of the problem that is worldwide neonatal mortality, it is paramount that all efforts are focused towards a community-based approach.
More research is needed to determine the efficacy of the community-based kangaroo method (CKMC). For now, studies do not seem to indicate that CKMC is feasible in remote or low-resource settings. One-randomized trial reported missing birth weights, a weak implementation, and no significant difference in mortality between the intervention and control groups (Sloan, 2008). However, evidence is limited and many more trials are required to truly assess the impact that CKMC could have in maternal and child health and determine the right formula to adopt (Barros, 2010).
Official protocols do not recommend the KMC approach for unstable preterm neonates. Nonetheless, a randomized trial on unstable newborns seems to indicate that KMC could also have significant risk reductions in mortality in that group (Bergman, 2015). These results are promising, but more similar studies are necessary to gauge the potential impact of KMC on unstable infants.
Conclusions
Overall, the implementation of KMC has led to a reduction in risk of mortality in LBW infants by 40%, a reduction in nosocomial infections by 58%, and savings of approximately US$570 per patient (Conde-Agudelo, 2014; Sharma, 2017). However, in the lowest wealth quintile of the world, more than 70% of all births are home deliveries (Montagu, 2011). The potential impact of facility-based KMC fades when keeping these numbers in mind. Furthermore, implementation of KMC in Africa and Southeastern Asia has been less than optimal. The SODOTO model of dissemination comes with good intentions, but generates vertical KMC programs that are too expensive to sustain (Bergman, 2015).
Coordinated actions and consistent communication among national and local leadership is needed to support essential health systems and financing requirements for KMC (Chan, 2017). Stakeholders must be involved in the discussion of ways to implement KMC in existing systems of care to provide sustainable treatment. Furthermore, research is required to evaluate the impact of community based KMC, where it could potentially reduce the most premature neonatal deaths.
Acknowledgments
We would like to sincerely thank Dr. Nathalie Charpak (Fundacion Canguro, Bogotà, Colombia) for her knowledge and guidance surrounding the implementation of KMC in Bogotà, as well as challenges experienced in the uptake of KMC elsewhere. Furthermore, we thank Dr. Madhu Pai and Emily MacLean (Department of Epidemiology and Biostatistics, McGill University, Montreal, QC) for their constructive comments on the writing of this report.
Appendix
| Outcomes | Conventional Neonatal Care | Kangaroo Mother Care | Relative Effect (95% CI) | Number of participants (studies) |
| Mortality at latest follow-up | 60 per 1000 | 40 per 1000 (29 to 57) | RR 0.67 | 2293 (12 studies) |
| Severe infection/sepsis at latest follow-up in stabilized infants | 30 per 1000 | 20 per 1000 (14 to 28) | RR 0.5 | 1463 (8 studies) |
| Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age in stabilized infants | 271 per 1000 | 76 per 1000 (43 to 133) | RR 0.28 | 989 (9 studies) |
| Weight gain at latest follow-up in stabilized infants | – | Mean weight gain (g/d) at latest follow-up in stabilized infants was 4.08 higher (2.3 to 5.86 higher) | – | 1198 (11 studies) |
| Any breastfeeding at discharge or at 40 to 41 weeks’ postmenstrual age in stabilized infants | 762 per 1000 | 914 per 1000 | RR 1.2 | 1696 (10 studies) |
| Any breastfeeding at 1 to 3 months’ follow-up in stabilized infants | 711 per 1000 | 832 per 1000 (747 to 932) | RR 1.17 | 1394 (9 studies) |
| Griffith quotient for psychomotor development (all subscales) at 12 months’ corrected age | – | Mean Griffith quotient for psychomotor development (all subscales at 12 months’ corrected age in the intervention groups was 1.05 higher (0.75 to 2.85 higher) | – | 579 (1 study) |
Table 1: Kangaroo Mother Care versus Conventional Neonatal Care for Reducing Morbidity and Mortality in LBW infants
*Adapted from the data tabulated in the Cochrane Systematic Review (Conde-Agudelo, 2016)
Courtney Fang is in her final year at McGill pursuing a BSc. in Microbiology and Immunology and a minor in International Development Studies. She is interested in infectious disease control, increasing access to healthcare, as well as maternal and child health particularly in LMIC. Courtney plans to go to graduate school next year to pursue her Master’s in Public Health.
Zoë Goldstein is a 5th year Psychology student interested in the applications of global mental health programs. She is also interested in psycho-oncology and the emotional lives of all folks dealing with chronic conditions and illnesses. She intends to go to graduate school for clinical psychology.
Morgan Kennedy is in her final year of the MSc. Public Health program at McGill University with an option in global health. She is pursuing a career in public health knowledge translation for improved participatory research and policy change.
Carla Achcar is in her final year at McGill completing a BSc. with a major in Pharmacology and a minor in Biotechnology. She has great interest in the development of optimized diagnostic tools that hold the potential to democratize access to healthcare. She is actively engaged in global health organizations on campus.
Bidjinie Coriolan is in her final year of the MSc. Public Health program at McGill University. She has a great interest in maternal and child health as well as non-communicable diseases. She is pursuing a career in program management and policy design.
Mackenzie Hamilton is a 4th year Honours Immunology student at McGill University, studying the adaptive immune responses to influenza virus-like-particle vaccines. She is passionate about infectious disease control and improving access to necessary treatments in low and middle income countries. She plans on continuing her education in immunology and hopes to be able to translate this work to the global health field in the future.
Edited by Benjamin Aloi
References
Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens C.E. (2010). Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy and Childbirth 10:S3.
Bergh, A., Arsalo, I., Malan, A. F., Patrick, M., Pattinson, R. C., & Phillips, N. (2007). Measuring implementation progress in kangaroo mother care. Acta Paediatrica, 94(8), 1102-1108. doi:10.1111/j.1651-2227.2005.tb02052.x
Bergh A.M., De Graft-Johnson J., Khadka N., Om’Iniabohs A., Udani R., Pratomo H., De Leon-Mendoza S. (2016). The three waves in implementation of facility-based kangaroo mother care: A multi-country case study from Asia. BMC International Health and Human Rights 16.
Bergman N.J. (2015). Kangaroo Mother Care in African countries. Acta Paediatrica 104:1208-1210.
Broughton, E. I., Gomez, I., Sanchez, N., & Vindell, C. (2013). The cost-savings of implementing kangaroo mother care in Nicaragua. Rev Panam Salud Publica, 34(3), 176-182.
Cattaneo A, et al. (1998). Recommendations for the implementation of kangaroo mother care for low birthweight infants. Acta Paediatrica, 87:440-445.
Chan, G. J., Labar, A. S., Wall, S., & Atun, R. (2015). Kangaroo mother care: a systematic review of barriers and enablers. Bulletin of the World Health Organization, 94(2). doi:10.2471/blt.15.157818
Chan, G. J., Valsangkar, B., Kajeepeta, S., Boundy, E. O., & Wall, S. (2016). What is kangaroo mother care? Systematic review of the literature. Journal of global health, 6(1).
Chan, G., Bergelson, I., Smith, E. R., Skotnes, T., & Wall, S. (2017). Barriers and enablers of kangaroo mother care implementation from a health systems perspective: a systematic review. Health Policy and Planning, 32(10), 1466-1475. doi:10.1093/heapol/czx098
Charpak, N., Ruiz-Pelaez, J.G., Figueroa de, C. Z., & Charpak, Y. (2001). A randomized controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics, 108(5), 1072-1079.
Charpak, N., Gabriel Ruiz, J., Zupan, J., Cattaneo, A., Figueroa, Z., Tessier, R., … & Mokhachane, M. (2005). Kangaroo mother care: 25 years after. Acta Paediatrica, 94(5), 514-522.
Charpak, N. & Ruiz-Peláez, J. G. (2007). Resistance to implementing Kangaroo Mother Care in developing countries, and proposed solutions. Acta Paediatrica, 95(5), 529-534. doi:10.1111/j.1651-2227.2006.tb02279.x
Charpak, N., Tessier, R., Ruiz, J. G., Hernandez, J. T., Uriza, F., Villegas, J., . . . Maldonado, D. (2017). Twenty-year Follow-up of Kangaroo Mother Care Versus Traditional Care. Pediatrics, 139(1). doi:10.1542/peds.2016-2063
Conde‐Agudelo, A., Belizán, J. M., & Diaz‐Rossello, J. (2016). Cochrane Review: Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Evidence‐Based Child Health: A Cochrane Review Journal, 7(2), 760-876
Darmstadt G.L., Bhutta Z.A., Cousens S., Adam T., Walker N., de Bernis L. (2005). Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet 365:977-988.
Every Newborn: an action plan to end preventable deaths (2014). Geneva: World Health Organization.
The Global Financing Facility 2016-2017 Annual Report (2017). Washington, DC: World Bank Publications.
Hall, D., & Kirsten, G. (2008). Kangaroo Mother Care – a review. TME Transfusion Medicine, 18(2), 77-82.
Lawn J.E., Kerber K., Enweronu-Laryea C., Cousens S. (2010). 3.6 Million Neonatal Deaths—What Is Progressing and What Is Not? Seminars in Perinatology 34:371-386.
Lawn J.E., Mwansa-Kambafwile, Horta B.L., Barros F.C., & Cousens, S. (2010). ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. International journal of epidemiology.
Lima G., Quintero-Romero S., Cattaneo A. (2000). Feasibility, acceptability and cost of kangaroo mother care in Recife, Brazil. Ann Trop Paediatr 20:22-26.
Martinez, H. The Mother Kangaroo Method. Innovation for Development and South-South Cooperation (IDEASS Colombia).
Rasaily, R., Ganguly, K., Roy, M., Vani, S., Kharood, N., Kulkarni, R., … Kanugo, L. (2017). Community based kangaroo mother care for low birth weight babies: A pilot study. The Indian Journal of Medical Research, 145(1), 51.
Reaching the Every Newborn National 2020 Milestones: country progress, plans and moving forward. (2017). Geneva World Health Organization
Rosero, L., Martinez, R. (n.d.) Implementation of KMC in Colombian Social Security. (n.d.). Fundacion Canguro. Retrieved December 12, 2017, from http://fundacioncanguro.co/encuentros/2encuentro/abstract16.htm
Ruiz, J. G., (1998). Kangaroo mother versus traditional care for newborn infants 2000 grams: A randomized control trial. Journal of Clinical Epidemiology: Supplement 1, 51, S12-S12.
Ruiz, J. G., Charpak, N., & Cuervo, L. G. (2004). Kangaroo Mother Care, an example to follow from developing countries. BMJ : British Medical Journal, 329(7475), 1179–1181.
Ruiz, J. G., Charpak, N., Castillo, M., Bernal, A., Ríos, J., Trujillo, T., & Córdoba, M. A. (2017). Latin American Clinical Epidemiology Network Series – Paper 4: Economic evaluation of Kangaroo Mother Care: cost utility analysis of results from a randomized controlled trial conducted in Bogotá. J Clin Epidemiol, 86 (Supplement C), 91-100. doi:https://doi.org/10.1016/j.jclinepi.2016.10.007
Seidman, G., Unnikrishnan, S., Kenny, E., Myslinski, S., Cairns-Smith, S., Mulligan, B., & Engmann, C. (2015). Barriers and Enablers of Kangaroo Mother Care Practice: A Systematic Review. PLoS ONE, 10(5), e0125643. http://doi.org.proxy3.library.mcgill.ca/10.1371/journal.pone.0125643
Sharma, D., Murki, S., & Oleti, T. P. (2017). Study comparing “Kangaroo Ward Care” with “Intermediate Intensive Care” for improving the growth outcome and cost effectiveness: randomized control trial. J Matern Fetal Neonatal Med, 1-8. doi:10.1080/14767058.2017.1359832
Shrestha, M. & Pokharel, N. (2005). Hypothermia in newborn babies. The Nursing Journal of India, 96(11), 255-256.
Simkiss, D. E. (1999). Kangaroo Mother Care. Journal of tropical pediatrics, 45(4), 192-194.
Wardlaw, Zupan, Ahman (2004). Low Birthweight: Country, Regional and Global Estimates. UNICEF.
World Health Organization. (2017). Preterm birth.
World Health Organization. (2003). Kangaroo mother care: a practical guide.
